|
|

|
|

|
|

|
|
Task 2 - Laboratory experiments
Task 2a - Partners in charge: D. Amouroux (IPREM-Pau, partner 4)
Reduction & bio-accumulation kinetics and isotopic fractionation of Se during the formation of
DMSe (DMS) by bacteria/plankton (D. Amouroux; E. Tessier; M. Bueno; M. Levasseur; C.
Cloquet).
This sub-task aims to better understand interactions between dissolved Se species and bacterio-plankton
by studying the reduction of Se(IV-VI) into Se(-II) and the formation of organometallic volatile molecules
(DMSe, DMDSe, ...). Isotopic fractionation factors related to these reactions will be determined. Indeed,
Se reduction reactions have shown important isotopic fractionation effects that might be expressed in the
environment (e.g. Johnson 2004). Results from this sub-task will be used for the interpretation of data
obtained from tasks 3 and 4.
Se bio-volatilization and isotopic fractionation experiments: Experiments will be conducted in the
laboratory (at IPREM-Pau, partner 4) in presence of bacterio-plankton cultures resistant to Se inorganic
concentrations from 10 to 100 μg/L. The bacterio-plankton will be selected from an available culture
collection of more than 50 strains of filamentous cyanobacteria from the Arctic and Antarctica (from W
Vincent, Partner 1). Most of these are in the order Oscillatoriales and are the main biomass constituents of
benthic microbial mats, complex microbial consortia that often dominate total ecosystem biomass and
productivity in high latitude lakes, ponds and rivers.
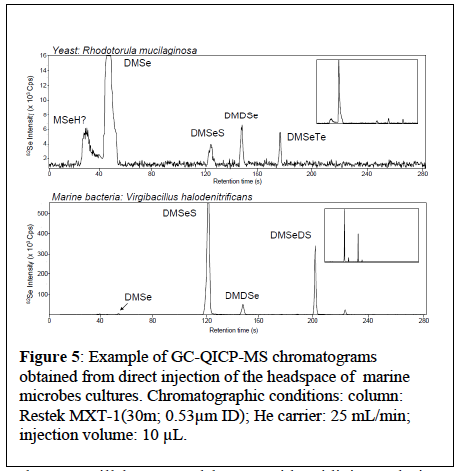
Se Biovolatilization: Selenite (SeO32-) and/or selenate (SeO42-) (to be determined as a function of the results of measurement campaigns) will be added to the cultures (six strains will be selected from available collection) in a stationary growing phase in an oxygenated but gas-tight environment. After 24- 48 hours of incubation, the dead volume of the culture will be sampled with a gas-tight syringe and directly injected and analyzed for Se gas components (DMSe, DMDSe), S components (DMS) and mixed components DMSSe) using a GC-QICP-MS. An additional preconcentration step by the mean of headspace solid phase microextraction (HSSPME) combined to the GC-QICP-MS technique has also been developed (Bueno & Pannier 2009) to improve the determination of the low concentrated volatile species. In the same way, issolved selenium speciation will be determined to identify potential precursors to the formation of volatile elenides. Figure gives example chromatograms of determination of volatile Se, Te and S species in direct headspace analysis of marine microbes pure cultures, by GC-QICP-MS.
Se isotopic fractionation: Experiments with higher yields of volatile Se
species will be selected for isotopic work. For
these, experiments will be re-conducted with
selenite and/or selenate previously
characterized for their Se isotopic
composition. This will be used as the initial
composition of the reaction for isotopic
fractionation factor calculations. Incubations
will be continuously degassed for 24h to 48h and gases will be trapped by an acid oxidizing solution
(HNO3/H2O2) in order to get in solution the Se volatile components. After Se purification, if needed
(Marin et al. 2003), this solution will be analyzed for its Se isotopic composition using the on-line hydride
generation MC-ICP-MS technique at CRPG-Nancy (partner 2) (Rouxel et al. 2002; Carignan and Wen
2007). Alternatively, purged gases will be stocked in adsorbent and/or cryogenic traps and directly
analyzed by GC-MC-ICP-MS after thermodesorption at IPREM-Pau (partner 4) (e.g. Amouroux et al.
1998 and in progress for the use of MC-ICP-MS).
Risks: the production of Se organic volatile molecules might be too small for isotopic measurements. Preconcentration
might be needed or, depending on the measured reaction kinetics, a longer gas purge time
might be necessary.





